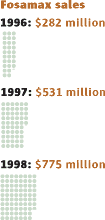
April 1984 – Experts at National Institutes of Health consensus conference endorse estrogen therapy and calcium supplements as ways to reduce bone loss. Over the next four years, sales of calcium supplements increase from $47 million to $200 million a year.
June 1992 – WHO invites experts to set international standards for screening patients for low bone density. They formally identify osteoporosis as a disease characterized by low bone mass and structural changes that lead to fracture risk. They also create a category of disease called osteopenia, or pre-osteoporosis.
1994 – WHO publishes recommendations that say "the argument for treating or screening all women is poor. ... The cost of preventive measures may possibly outstrip the savings made by preventing fractures."
March 9, 1995 – In a meeting with Food and Drug Administration officials, executives from the drug maker Merck & Co. discuss Fosamax, a new osteoporosis medication they are planning to submit for approval.
March 31, 1995 – Merck files a new-drug application with the FDA for Fosamax.
August 1995 – Merck creates the Bone Measurement Institute as a nonprofit subsidiary to increase the use of bone-measuring machines in doctors' offices.
September 1995 – FDA approves Fosamax, marketing begins.
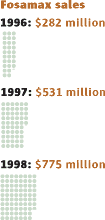
1999 – WHO panel recommends ways to measure osteoporosis burden on health systems but fails to disclose that eight of the 11 panelists were employed by drug companies.
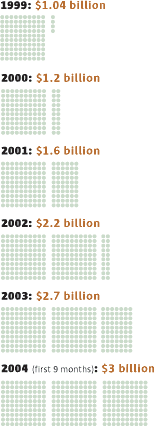
Sources: Food and Drug Administration, World Health Organization, Securities and Exchange Commission, Preventive Services Task Force, Science magazine