Originally published June 28, 2005 at 12:00 AM | Page modified March 16, 2010 at 4:54 PM
Disease expands through marriage of marketing and machines
Every day in clinics and doctors' offices across the country, healthy middle-age women slide their wrists into portable X-ray machines that...
Seattle Times staff reporter
BETTY UDESEN / THE SEATTLE TIMES
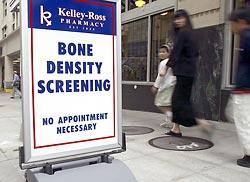
A sidewalk sign at Kelley-Ross Pharmacy in Seattle advertises bone-density screening. Such screening has proliferated in recent years, targeting younger, healthier people.
GREG GILBERT / THE SEATTLE TIMES
Joy Lewis, left, gets a bone-density scan at the Washington Athletic Club in downtown Seattle as Bobette Jones explains what the test results will mean. Jones, who owns the portable machine with several other investors, had charged about $25 and now charges $45 per scan. She travels the state, offering scans to adults of all ages at health fairs, businesses and government offices. In addition to T-scans she gives exercise and nutrition advice.
Every day in clinics and doctors' offices across the country, healthy middle-age women slide their wrists into portable X-ray machines that calculate bone density.
If they get a low enough density score, they walk out with a prescription that's supposed to prevent a hip fracture late in life by adding bone tissue now.
But there's a big problem with this familiar exercise, according to some top osteoporosis experts: Most of these women don't need the drug. They are wasting money and risking side effects for little benefit.
"If you're a healthy 50-year-old — an average woman going through menopause — your chances of getting a fracture ... are very low," said Dr. Susan Ott, a bone specialist at the University of Washington. "Yet they are pushing the drug right at that age group."
By targeting women in their 50s, manufacturers of drugs for osteoporosis have helped transform osteoporosis from an underrecognized disease in elderly women into what some say is a disease affecting tens of millions.
Drug companies and advocacy groups accomplished that by:
• Expanding the disease to include a new condition, osteopenia, or pre-osteoporosis, with boundaries so broad they include more than half of all women over 50.
• Promoting osteopenia and osteoporosis directly to these younger, healthier women, telling them they are at risk and should consider taking bone-strengthening drugs such as Fosamax.
• Shaping the way osteoporosis and osteopenia are defined, with readings from bone-density machines that the drug industry promoted, subsidized and helped put in doctors' offices.
All of this was done in the name of prevention. But Dr. Steven Cummings, one of the world's experts on osteoporosis and the director of the San Francisco Coordinating Center, a research center, says the drug companies' push has been driven by marketing as well as medicine.
"The word 'prevention,' which has become so popular, has also created problems," Cummings said.
"Drug therapy for women with osteopenia does do some good because it reduces the risk of spine fractures, but women with osteopenia have a low risk of those fractures. ...
"So taking a drug for osteopenia probably does not improve the quality of life for women with osteopenia. It does generate huge sales."
For elderly women who suffer fractures, bone drugs such as Fosamax are life-enhancing: strengthening bone, reducing chances of future breaks.
The bone-density X-ray machines promoted by Merck & Co. and other drug companies also are powerful advances in treating the disease. Doctors can use them to diagnose those truly at risk and track their recovery.
But, as Cummings points out, the utility of the machines and the drugs for middle-age women is unproven, unless they've had fractures previously. Their growing use is another cautionary tale of the influence of drug companies on the definition and treatment of disease.
Even a former lobbyist for Merck concedes that the company's focus has been on expanding the market for Fosamax, also known by its chemical name, alendronate.
"The goal is to make the uses as broad as humanly possible," said Kurt Furst, who worked for Merck from 1997 to 2000. "Merck would tell you virtually any woman post-menopausal should go on Fosamax. That's a heck of a lot of women."
By focusing on women 50 and older, Merck is following what other health organizations recommend, a company spokesman, Skip Irvine, said.
The numbers game
Experts devise a numerical measurement of bone density — and the boundaries of a new condition.
A generation ago, doctors diagnosed osteoporosis only "after the fall" — once an elderly patient broke a hip or developed a "spinal hump." There were few effective treatments for the crippling, costly condition.
As women began living longer, frustrated physicians saw more and more cases. In 1984, the National Institutes of Health (NIH), the federal medical-research agency, sponsored a conference of bone experts to discuss possible ways to prevent osteoporosis.
Scientists knew that adding calcium to diets could build bone. What they did not know: whether adding thickness alone would reduce the chances of fractures later on.
Bone strength, it would turn out, depended not just on density but on heredity and "bone quality" — the shape and number of spindly bone-cell connections inside the bones.
Experts at the conference opted to focus on bone size, the only aspect of bone health with existing treatments. They recommended that women consume more calcium. That decision "turned out to be quite a big industry," said Dr. Lawrence Shulman, a former NIH institute director who organized the conference.
Sales of calcium supplements skyrocketed. Media interest intensified. And drug companies, recognizing the market opportunity, began developing more drugs to increase bone density.
They supported new medical societies focused on osteoporosis and pumped money to doctors and scientists for research.
Meanwhile, the World Health Organization (WHO), the medical agency of the United Nations, decided to figure out if health-care systems could save money by preventing fractures instead of paying to treat them after they occurred.
The WHO sponsored an osteoporosis conference in Rome in 1992, partnering with the International Osteoporosis Foundation, a nonprofit organization with a corporate advisory board that currently consists of 31 drug and medical-equipment companies.
The central issue of the conference was whether osteoporosis could be identified before a patient broke a bone, not after. That required a different way of looking at the condition, a definition based on a numerical measurement of bone density.
But what would the magic number be? Everyone loses bone mass as they age. How would "normal" be defined, and what would be the threshold for prescribing drugs?
Experts at the conference, which was sponsored by two large drug companies and a drug-company foundation, decided that "normal" would be the bone density of a woman at age 30, roughly the age when bone mass peaks for most people.
Any difference between a woman being measured and the established standard would be reported as a "T-score." The T-score of a 70-year-old would reflect a comparison to a woman 40 years younger.
The next step was even trickier: How far below normal would density have to fall before it was considered osteoporosis? What T-score would define the disease?
The researchers turned to an analysis of women in Rochester, Minn., that showed about 16 percent of post-menopausal women in that city would sustain a hip fracture in their lifetime. Looking at years of bone-density scores, the WHO committee calculated that 16 percent of post-menopausal women had bone-density readings of -2.5 or worse.
So under the new definition, anyone with a spinal fracture or a -2.5 T-score or worse had osteoporosis, the doctors decided. That score roughly translates into bones that are 32 percent less dense than those of the average 30-year-old woman.
The WHO committee went even further. It said scores between -1 and -2.5 were the boundaries of a new condition, "osteopenia," or low bone mass.
In a single conference, one disease — osteoporosis — had been expanded from an elderly person with a fracture to anyone who had a -2.5 T-score. And another condition, osteopenia, was created.
An important result: Doctors could bill insurance for the bone-density test, which insurance typically had not covered.
Not everyone looked favorably on the developments.
Dr. Leo Lutwak, a retired U.S. Food and Drug Administration scientist who attended the conference, had argued against creating a diagnosis of osteopenia. He feared that the condition would be used incorrectly to label patients as having a disease, making it easier to treat them with new bone drugs.
The National Osteoporosis Foundation, another nonprofit backed by drug firms, estimates that 10 million Americans now have osteoporosis and that the disease is "a threat for an estimated 44 million Americans, or 55 percent of people 50 years of age and older."
With osteopenia, what patients had were measurements, not disease, said Cummings, the San Francisco expert.
"A lot of people who have an average risk of fracture for their age are told they have a disease," he said. "The average person doesn't know how that came to be or what it really means. But what some women worry what this means is you're at risk of falling apart. You're going to break everything. Doctors began to believe that's what it means."
Machines expand market
Merck pushes bone-measurement technology into doctors' offices.
In 1995, the FDA approved the much-anticipated Fosamax, which essentially adds mineral to bone, fossilizing it and making it harder. The drug's possible side effects include upper gastrointestinal irritation, ulcers of the esophagus, skin rash, low blood calcium and, in rare instances, necrosis of the jaw.
Merck launched a marketing juggernaut around bone-density testing. Marrying machine to medicine, Merck promoted portable bone-measuring devices that doctors could use in their offices to identify those with bone loss.
The company also bought the exclusive rights to one company's bone-testing technology, gave a loan to another company to help develop a different machine, and financed two other firms in order to increase the number of machines in doctors' offices.
Merck also created the Bone Measurement Institute, a nonprofit subsidiary with the goal of increasing the use of density-testing machines.
The goal wasn't only to sell the drug to the elderly who had osteoporosis. Merck officials said they were aiming for the 40 million post-menopausal women in America, according to a Columbia University Business School study of the company.
"They intended to turn Fosamax into a primary care product in the long run," the study said.
When Merck started its push in 1995, there were 750 bone-measuring devices in the United States. By 1999, there were between 8,000 and 10,000, according to Merck.
"The drug companies started to make machines available to doctors' offices and enter into big agreements to reimburse them for scans," said Dr. Andrea LaCroix, senior investigator for the Center for Health Studies at Group Health Cooperative in Seattle.
"We were all keenly aware of it. If you were in clinical practice, you could get the machines relatively cheap."
Doctors found themselves pressured to provide the density tests.
"From the marketing point of view, if you're a provider, you're facing a groundswell of demand for testing," LaCroix said. "We know it's not doing a lot of good, but we can't deny them because" otherwise the patients would "go someplace else."
The number of people tested on the machines rose to about 3.5 million a year, a Merck official said.
FDA warns Merck

The U.S. Food and Drug Administration warned Merck in 1997 to stop implying that all women develop osteoporosis at menopause.
In its letter, the FDA noted that twice before it had advised the company about proper wording.
In 2001, the FDA warned Merck that its Fosamax Web site "overstates the benefits while minimizing the risks associated with the drug."
![]() 1997 and 2001 letters [PDF]
1997 and 2001 letters [PDF]
![]() "Compare the facts" campaign [PDF]
"Compare the facts" campaign [PDF]
Merck targeted ads and brochures directly at younger women, telling them that "menopause is the single most important cause of osteoporosis."
But the FDA ordered the company to stop using that phrase in its ads. In a July 1997 letter, the agency told Merck that the claim "overstates the population eligible for therapy with Fosamax by implying that all women develop osteoporosis at menopause."
Meanwhile, Merck's lobbyists helped to persuade Congress to pass the Bone Mass Measurement Act in 1997. It authorized Medicare to reimburse doctors for performing bone-density tests, opening the door to coverage by other insurers.
Merck was so successful in marrying its marketing to the measurement that its Fosamax campaign was adopted by the industry as a "best-practices" model for other drug companies looking to expand their markets.
Skepticism grows
Various machines mean varying results, critics say.
But skepticism quietly took hold in some corners of the medical community.
"It's a violent storm in a very small puddle," said Dr. Brian Garra, a professor of radiology at the University of Vermont who chaired a gathering of experts who talked about scrapping the T-score measure in 1999, even though many of them had long associations with drug companies making osteoporosis treatments.
They were concerned about the reliability of the measures provided by so many different machines with varying standards and accuracy. A person could be measured on different machines and come up with widely varying T-scores. The small, portable machines that tested density at the wrist were not as reliable as the large machines known as central DEXA, studies showed.
The system of diagnosing osteoporosis with T-scores was in danger of falling apart. The main reasons for keeping it, it seemed, hinged on the fact that it had become so entrenched in the medical culture.
Merck's Bone Measurement Institute director said as much during a FDA hearing in May 1999.
"We understand that the T-score is not an ideal measurement, but it serves many, many valuable purposes," Dr. Lewis Sherwood told the assembled experts. "Even more importantly, it is embedded so thoroughly in many processes that are used widely."
Dennis Black, a University of California, San Francisco statistician, told the panel: "The manufacturers as well as the pharmaceutical companies have been very successful in promulgating T-scores. So I think there is a feeling that we can't abandon those T-scores totally."
The experts at the FDA hearing agreed a better way than T-scores was needed to assess someone's risk for fractures. They also agreed that women were being prescribed drugs they didn't need.
"If you abandon the one string that people hold onto, there will be nothing to hold onto, and the disease won't be treated at all," Black said in an interview. "It's better for people to do the wrong thing or not optimal thing than to do nothing."
Looking for a better way
Task force would reduce bone testing, rely on "evidence-based medicine."
Black and others are trying to move the field toward a measure of "absolute fracture risk." Instead of just a T-score, a woman would be told the likelihood, stated as a percent, of breaking her hip in the next five years, given her age, race and overall health.
For example, for a healthy, white 50-year-old woman with osteopenia, the risk of a hip fracture over the next five years would be less than 1 percent. Her lifetime risk for a hip fracture would range from 16 percent to 27 percent.
But doctors need to be careful about not scaring patients with new numbers, Cummings said. "We need to make sure people understand that risk, not just that they receive another number," he said.
Today, many physicians, scientists and osteoporosis experts are pushing hard to scale back bone testing.
Many of them embrace what is called "evidence-based medicine." It relies less on treatment guidelines from expert opinions and more on empirical studies based on tests, medical data and outcomes from thousands of patients.
In 2002, a federal committee chaired at the time by Dr. Al Berg, head of the University of Washington's Department of Family Medicine, developed recommendations that cut through corporate marketing and focused instead on evidence for screening.
The committee was part of the U.S. Preventive Services Task Force, which bars members from having financial ties to drug makers. Its recommendations are highly regarded in primary care and preventive medicine.
The committee recommended that bone testing be sharply targeted. Women 65 or older should be tested, as well as those over 60 who weighed less than 127 pounds and were not taking estrogen replacement. Testing should be done at the hip with a DEXA machine, the committee said.
The recommendations gave credence to those experts who had become concerned that millions of women would be exposed to drugs for decades at great expense and without evidence that the drugs were safe or effective for them.
Experts also said that some elderly women who need the drugs might not be getting them.
A recent study of 459 patients above age 60 who got chest X-rays in one hospital emergency room in Canada showed that one in six had spinal fractures that indicated osteoporosis.
But in nearly half of those cases, radiologists didn't spot the fractures or note them in their reports. Only 25 percent of the patients were treated for osteoporosis, the study showed.
As result, said Ott, the UW bone specialist, women who could have benefited from drugs such as Fosamax weren't getting treated for the disease.
"What's happening is these women who need it are still being terribly ignored," Ott said.
"Meanwhile, the women in the advertising look like they're about 40 years old."
Send comments to suddenlysick@seattletimes.com or call Susan Kelleher: 206-464-2508.
Entertainment | Top Video | World | Offbeat Video | Sci-Tech
general classifieds
Garage & estate salesFurniture & home furnishings
Electronics
just listed
More listings
POST A FREE LISTING